The DREAMM clinical development program
Investigating BCMA-targeted antibody-drug conjugate therapy in multiple myeloma
Clinical trials
4th Line combination study
A Phase 1/2, Randomized, Open-label Platform Study Utilizing a Master Protocol to Study Belantamab Mafodotin as Monotherapy and in Combination With Anticancer Treatments in Participants With Relapsed/Refractory Multiple Myeloma1,2
2nd Line combination study
A Multicenter, Open-Label, Randomized Phase 3 Study to Evaluate the Efficacy and Safety of the Combination of Belantamab Mafodotin, Bortezomib, and Dexamethasone (B-Vd) Compared With the Combination of Daratumumab, Bortezomib and Dexamethasone (D-Vd) in Participants With Relapsed/Refractory Multiple Myeloma3,4
2nd Line combination study
A Phase 3, Multicenter, Open-Label, Randomized Study to Evaluate the Efficacy and Safety of Belantamab Mafodotin in Combination With Pomalidomide and Dexamethasone (B-Pd) Versus Pomalidomide Plus Bortezomib and Dexamethasone (PVd) in Participants With Relapsed/Refractory Multiple Myeloma5,6
1st Line combination study
A Phase 1, Randomized, Dose and Schedule Evaluation Study to Investigate the Safety, Pharmacokinetics, Pharmacodynamics and Clinical Activity of Belantamab Mafodotin Administered in Combination With Standard of Care in Participants With Newly Diagnosed Multiple Myeloma7
1st Line combination study
A Study of Belantamab Mafodotin Administered in Combination With Lenalidomide and Dexamethasone (BRd) Versus Daratumumab, Lenalidomide, and Dexamethasone (DRd) in Participants With Newly Diagnosed Multiple Myeloma (NDMM) Who Are Ineligible for Autologous Stem Cell Transplantation (TI-NDMM) (DREAMM-10)8
3rd Line monotherapy study
A Phase 1 Study to Evaluate the Pharmacokinetics and Safety of Belantamab Mafodotin Monotherapy in Participants With Relapsed or Refractory Multiple Myeloma Who Have Normal and Varying Degrees of Impaired Renal Function9
3rd Line monotherapy study
A Phase 1 Study to Evaluate the Pharmacokinetics and Safety of Belantamab Mafodotin Monotherapy in Participants With Relapsed or Refractory Multiple Myeloma Who Have Normal and Varying Degrees of Impaired Hepatic Function10
4th Line monotherapy study
A Phase 2, Randomized, Parallel, Open-label Study to Investigate the Safety, Efficacy, and Pharmacokinetics of Various Dosing Regimens of Single-agent Belantamab Mafodotin in Participants With Relapsed or Refractory Multiple Myeloma11,12
4th Line combination study
A Study to Investigate the Safety and Efficacy of Belantamab for the Treatment of Multiple Myeloma When Used as Monotherapy and in Combination Treatments (DREAMM-20)13
Mechanism of action
Belantamab mafodotin is a BCMA-targeted antibody-drug conjugate with a humanized anti-BCMA mAb conjugated to the cytotoxic payload mafodotin14
About multiple myeloma and BCMA
Multiple myeloma is characterized by the uncontrolled growth of malignant plasma cells. BCMA, a membrane protein, is expressed on all multiple myeloma cells and supports myeloma cell proliferation and survival. Belantamab mafodotin was designed to specifically bind to BCMA and eliminate myeloma cells by a multimodal mechanism.14-16
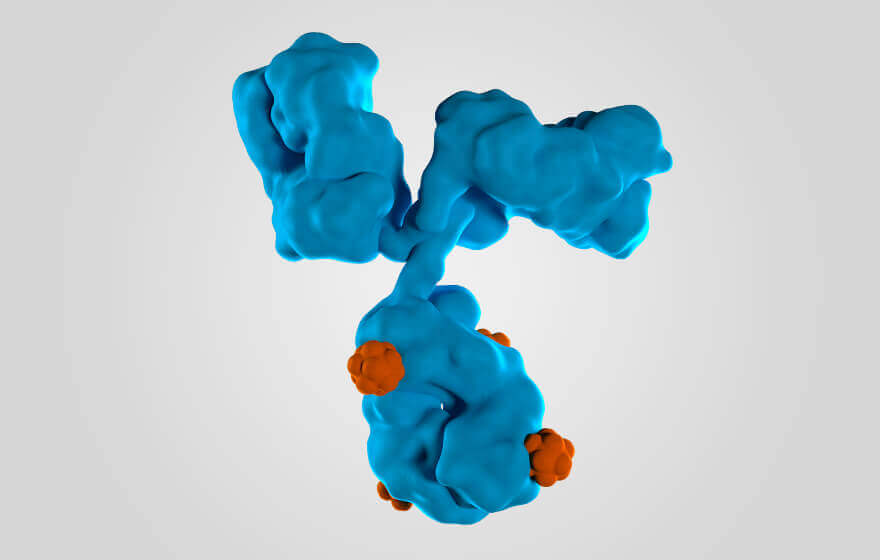
BCMA, B-cell maturation antigen; mAb, monoclonal antibody.
